Our Process Is
Different
Anybody can build a prototype, we build companies
Our Team combines decades of clinical experience with medical device sales, marketing and product development expertise. We evaluate projects based on business development potential and then develop a comprehensive strategy, including design, intellectual property, regulatory, and market development customized to the exit goal. Let us show you how our unique process of evaluation and strategic planning can help to de-risk your project and increase the chance of a successful exit.
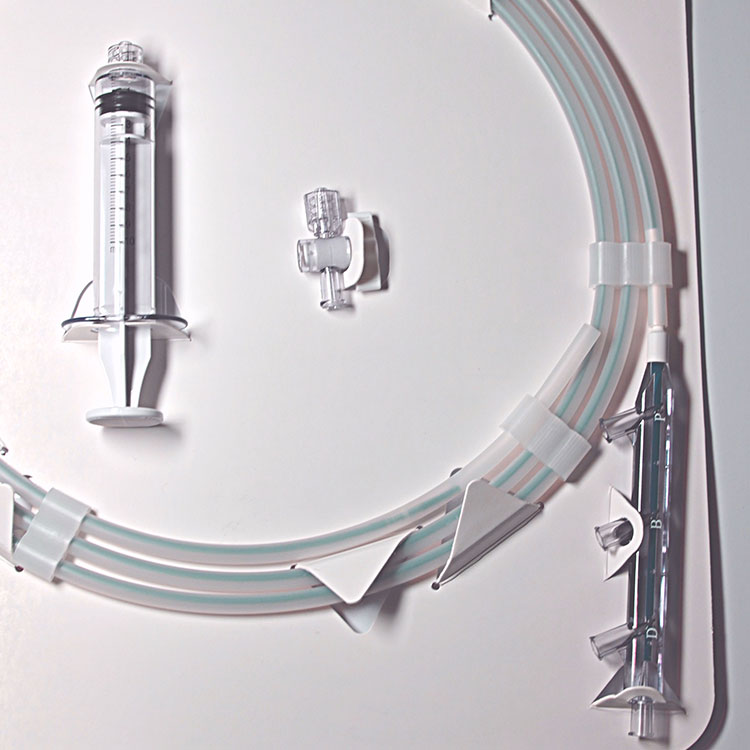
Our Story
We founded EndoRx to change how early stage medical device technologies are brought to market.
Our first product, the Rhino, was the first biliary stone removal balloon with an expansion range of 9-22mm, eliminating the need for traditional balloon size selection by conforming to any size bile duct. We successfully navigated the Rhino from concept to 510k clearance in 13 months.
EndoRx launched the Rhino at DDW 2015 and sold it from our office in the Texas Medical Center until ConMed acquired the product line in the spring of 2017. EndoRx now develops medical technologies both organically and as a full-service management consulting firm with outside partners to bring physician driven innovation to market
Our Projects
EndoRx maintains an organic pipeline of disruptive Endoscopic Technologies
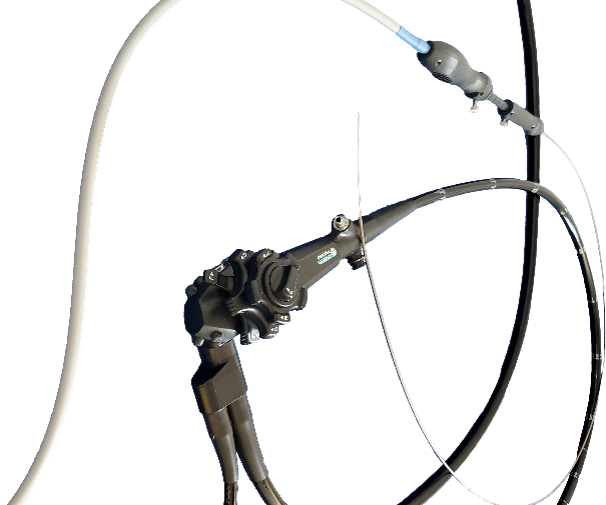
Rhino
The first “one size fits all option” biliary stone retrieval balloon with 9-22MM expansion capability in one balloon.
Exited to ConMed April 2017.
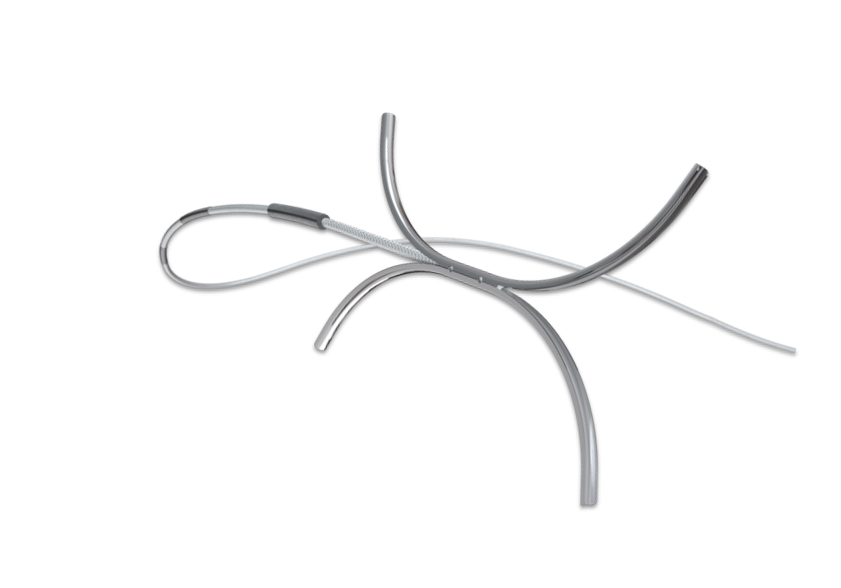
ADB
The first independently controlled dual anchoring dilation system designed to eliminate balloon slip for all GI procedures.
Our Clients
EndoRx is proud to call some of the most exiting start ups in medical device space our partners.



![gic-product-3 [1]](https://endorxmedical.com/wp-content/uploads/2022/04/gic-product-3-1.png)


Comprehensive Development Solutions
Our Services
It’s time to get out of R&D land….
Project Management
We provide a unique combination of seasoned executive business leadership with top tier scientific advisory board consulting.
Learn More >
Project Management
- Prototype development
- Manufacturing vetting and selection
- Commercialization project management
- Quality system development
Market Development
We plug you into our global network of Key Opinion Leaders and Industry consultants.
Learn More >
Market Development
- KOL meetings and SAB recruitment
- Marketing resource development
- Connection with key industry leaders
- Trade show solutions and management
Strategic Engagement
We have extensive relationships with industry strategics across multiple specialties.
Learn More >
Strategic Engagement
- Custom slide deck creation
- Market modeling and valuation analysis
- Intro meetings with industry stake holders
- Negotiation management
The Latest Endo Rx
News
Announcing Novascan’s Launch of a Human GI trial for its nsCanary™ Cancer Detection Device
NovaScan, a clinical stage oncology company based in Chicago, announced that it has started a human GI clinical trial for its nsCanary™ device at Texas International Endoscopy Center (TIEC), led by Principal Investigator Isaac Raijman MD. This trial will include 200 patients across several clinical areas, including upper and lower gastrointestinal lumen, pancreas and biliary tract. It is anticipated that the trial will collect the data by the Spring of 2023.
Abstract on FrostBite™ EUS-Compatible Cryocatheter Accepted for Presentation at the American College of Gastroenterology 2022 Annual Meeting
GI Cryo, Inc., a preclinical medical device development spin-off company from CPSI Biotech, will be presenting on the development and evaluation of the novel FrostBite™ endoscopic ultrasound (EUS) cryocatheter at the annual meeting for the American College of Gastroentrology (ACG2022) this October.
NovaScan Invited for Poster Presentation at ACG Annual Meeting
NovaScan, a clinical stage oncology company based in Chicago, announced that it will be presenting a poster at ACG Annual Scientific Meeting, to be held from October 21-26th at the Charlotte Convention Center in Charlotte NC.







